Lewis Structure of SO4 2 (Sulphate ion) YouTube
SOLVED Draw the Lewis Structure for SO4 2* to complete the following SO4 2 has sigma (0) bonds
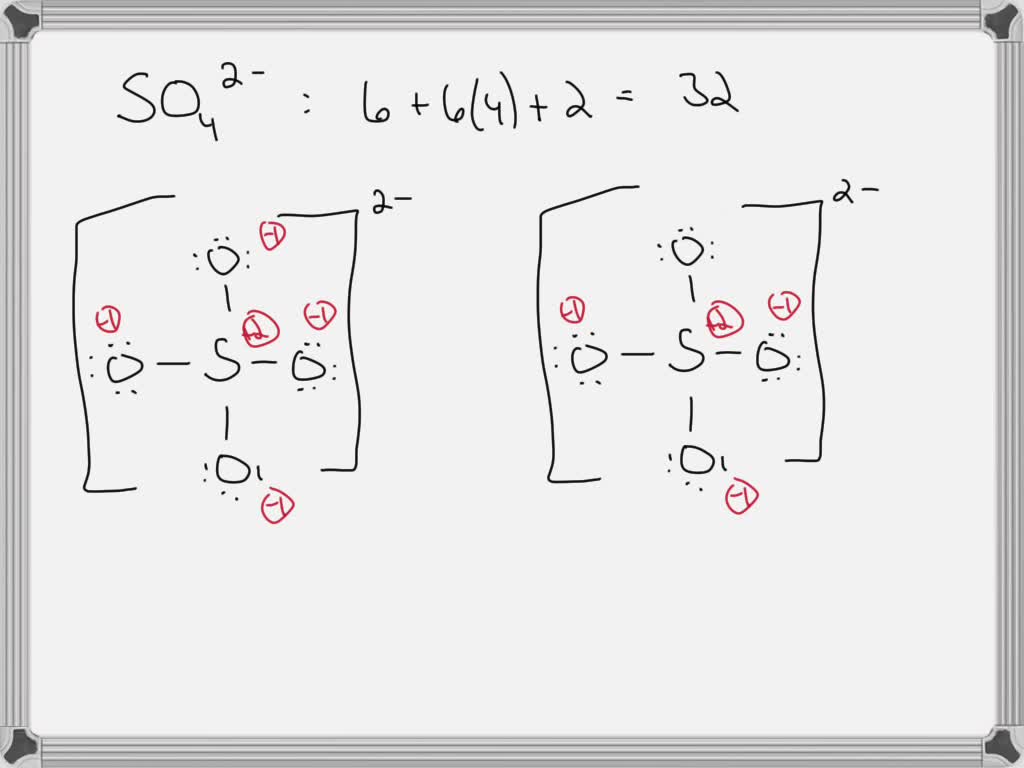
The second step in sketching the SO 4 2-Lewis structure involves determining the total number of electron pairs. With 32 valence electrons present in SO 4 2-, the total number of electron pairs can be found by dividing this value by two.This results in a total of 16 electron pairs.. The third step in sketching the SO 4 2-Lewis structure is to determine the central atom.
Electron dot structure of the sulfate ion SO42 52 Chemistry Net
5 Steps to Draw the Lewis Structure of SO4 2- ion Step #1: Calculate the total number of valence electrons. Here, the given ion is SO4 2-.In order to draw the lewis structure of SO4 2-ion, first of all you have to find the total number of valence electrons present in the SO4 2-ion. (Valence electrons are the number of electrons present in the outermost shell of an atom).

Number of Lone Pairs and Bonding Pairs for SO4 2 (Sulfate ion) YouTube
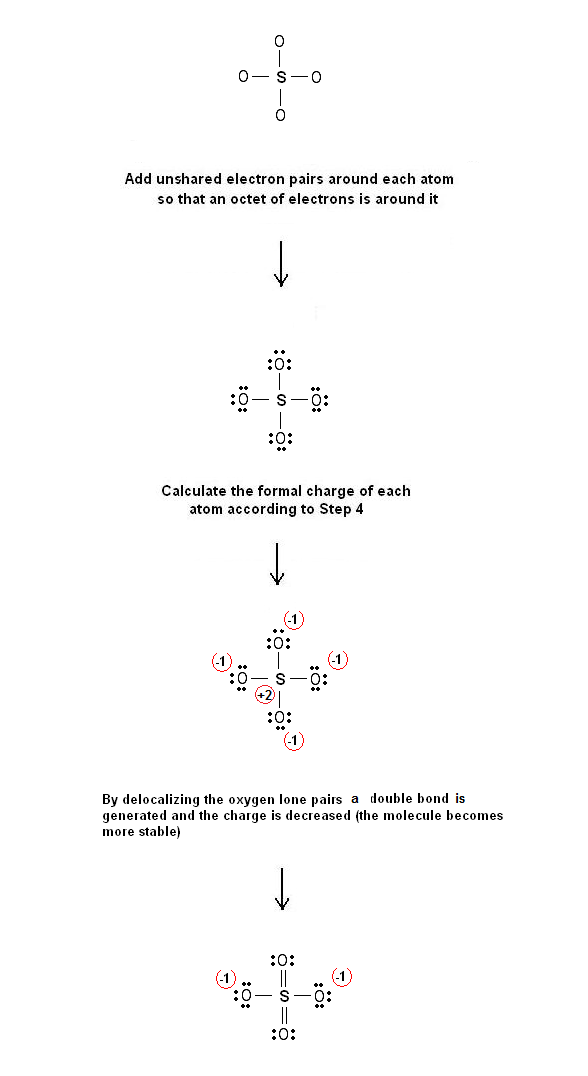
Sulfur make six bonds in this Lewis Structure. Two of the oxygens are single-bonded and two are double-bonded. The reason is FORMAL CHARGE and the fact tha.

Hướng dẫn vẽ cấu trúc Lewis của so4 2 lewis structure chi tiết và dễ hiểu
Let's do the SO4 2- Lewis structure, for the sulfate ion. On the periodic table: Sulfur, 6 valence electrons; Oxygen also has 6, we have 4 Oxygens, multiply by 4; and these 2 valence electrons up here, we need to add those, as well. That gives us a total of 32 valence electrons. We'll put the Sulfur in the center, and then the four Oxygens will.
how to draw lewis structure for so4 2
16 S dengan konfiguasi 2, 8, 6 memiliki kecenderungan untuk menangkap 2 elektron. Sedangkan 8 O dengan konfigurasi 2, 6 memiliki kecenderungan juga untuk menangkap 2 elektron. Sehingga seharusnya ketika S berikatan dengan O akan membentuk SO, sesuai kebutuhan. Namun ketika membentuk SO 2, maka atom S akan mengikat 2 atom O, dimana yang satu berikatan kovalen sejati sedangkan atom O yang lain.

Lewis Dot Diagram For So4 2
A step-by-step explanation of how to draw the Sulfate Ion Lewis Dot Structure (SO42- ). We'll also look at the molecular geometry, bond angles, electron geo.

Lewis Structure of SO4 2 (Sulphate ion) YouTube
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

lewis structure of SO42 Brainly.in
Dalam CH 4 terdapat empat pasang elektron ikatan, sehingga terdapat 4 garis atau lengan ikatan, bisa dilihat pada gambar berikut: Struktur Lewis CH 4 menggunakan garis. Ikatan kovalen yang terbentuk pada senyawa CH 4, dinamakan ikatan kovalen tunggal, karena terdapat empat ruas garis tunggal antara atom C dan H. Agar lebih memahami pembentukan.
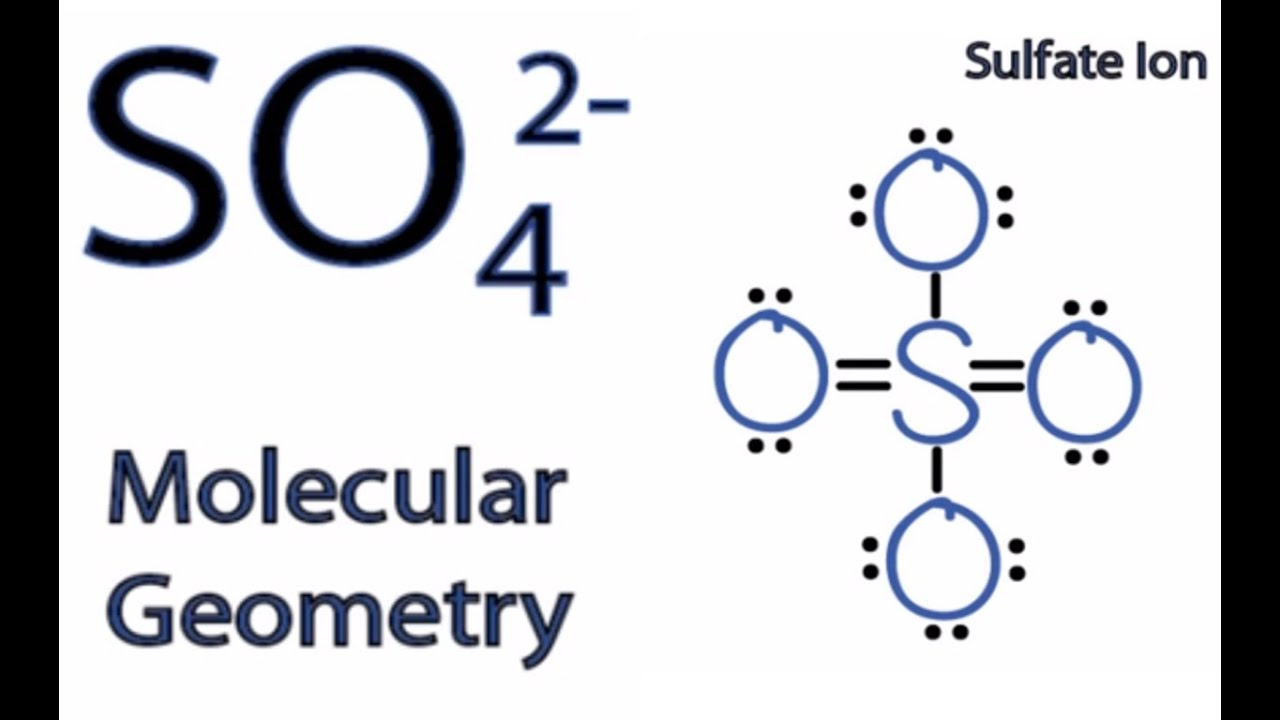
SO4 2 Molecular Geometry / Shape and Bond Angles YouTube
I quickly take you through how to draw the Lewis Structure of SO4 2- (Sulfate Ion) . I also go over hybridization, shape and bond angles.

Struktur Lewis Asam Sulfat H2SO4 dan Cara Menggambarnya
SO42- Lewis Structure, Hybridization, Bond Angle and Molecular Geometry. SO42- is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of -2. It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more.

Estructura de Lewis del (SO4)2 y del (CO3)2 YouTube
A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion).For the SO4 2- structure use the periodic table to find the total numb.
[Solved] For SO4^2 sulfate ion, draw the Lewis structure by counting... Course Hero
Drawing the Lewis Structure for SO 42- ( Sulfate Ion) Sulfates (salts with the SO 42-) are frequently used in industry and biologically. A commonly used sulfate is sodium lauryl ether sulfate found in shampoo, toothpaste, etc. For example, MgSO 4 is also known as Epsom Salts. There are 32 valence electrons available for the Lewis structure for.

Bentuk Molekul SO4 2 MateriKimia
Step 2: Select the central atom. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Now here the given ion is SO4 2- ion and it contains sulfur atom (S) and oxygen atoms (O). You can see the electronegativity values of sulfur atom (S) and oxygen atom (O) in the above periodic.

SO3 2 Lewis Structure How to Draw the Lewis Structure for SO3 2 (Sulfite Ion) YouTube
The SO4 2- (Sulfate Ion), comprised of one sulfur atom and four oxygen atoms, presents a captivating example of a chemical species with intriguing properties. At the heart of comprehending the characteristics and reactivity of SO4 2- lies the exploration of its Lewis structure. Through a combination of explanations, visual aids, and frequently asked questions,

Hướng dẫn vẽ cấu trúc Lewis của so4 2 lewis structure chi tiết và dễ hiểu
Lewis Dot of the Sulfate Ion. SO 42-. Back. 70 More Lewis Dot Structures. S does not follow the octet rule. It will hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, will also have access to the 3d sublevel, thus allowing for more than 8 electrons. Elements in the first 2 periods of the Periodic Table do not.

Lewis Structure ( SO4 2 ) YouTube
There are equivalent six resonance structures SO4 2- the Sulfate ion. We start with a valid Lewis structure and then follow these general rules.- Resonance.