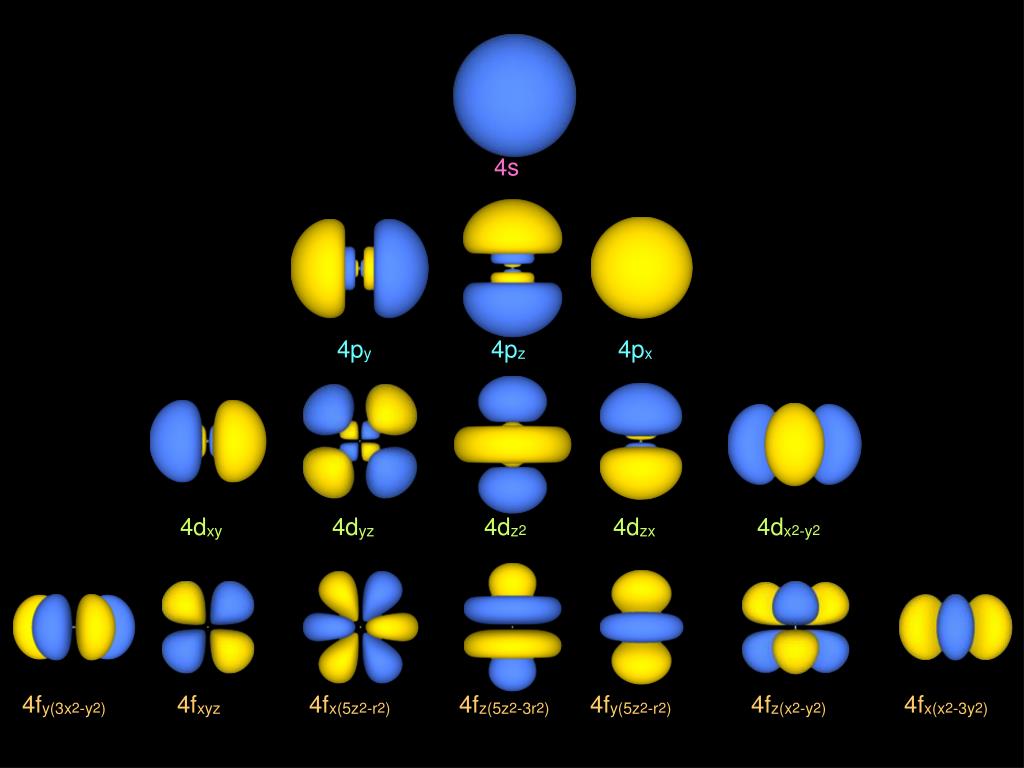
6.6 3D Representation of Orbitals Chemistry LibreTexts
PPT Atomic orbital & Hydrogenatom wave function PowerPoint
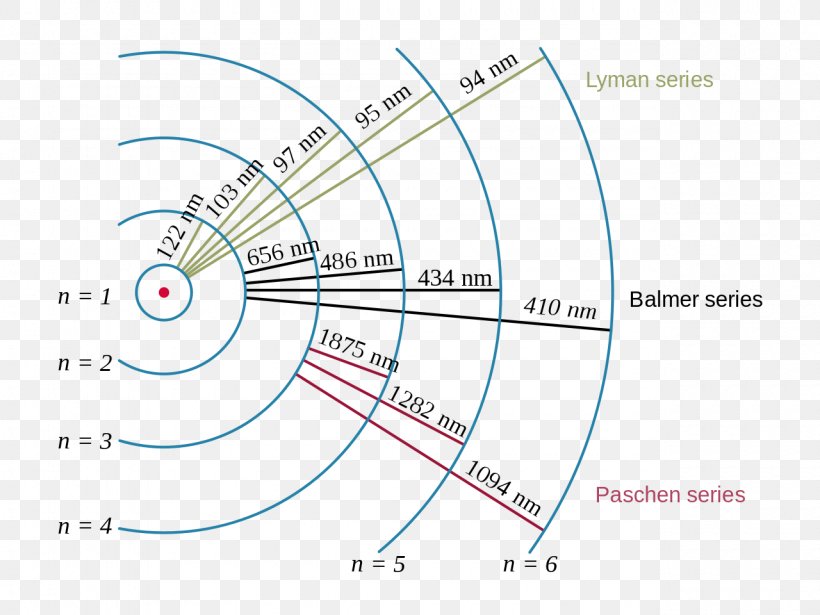
Figure \(\PageIndex{6}\): Energy-level diagram for hydrogen showing the Lyman, Balmer, and Paschen series of transitions. The orbital energies are calculated using the above equation, first derived by Bohr. We see that Bohr's theory of the hydrogen atom answers the question as to why this previously known formula describes the hydrogen spectrum.

Diagram Representation Of The Element Hydrogen Stock Vector Image
Figure 8.2.1: A representation of the Bohr model of the hydrogen atom. With the assumption of a fixed proton, we focus on the motion of the electron. In the electric field of the proton, the potential energy of the electron is. U(r) = − ke2 r, where k = 1 / 4πϵ0 and r is the distance between the electron and the proton.
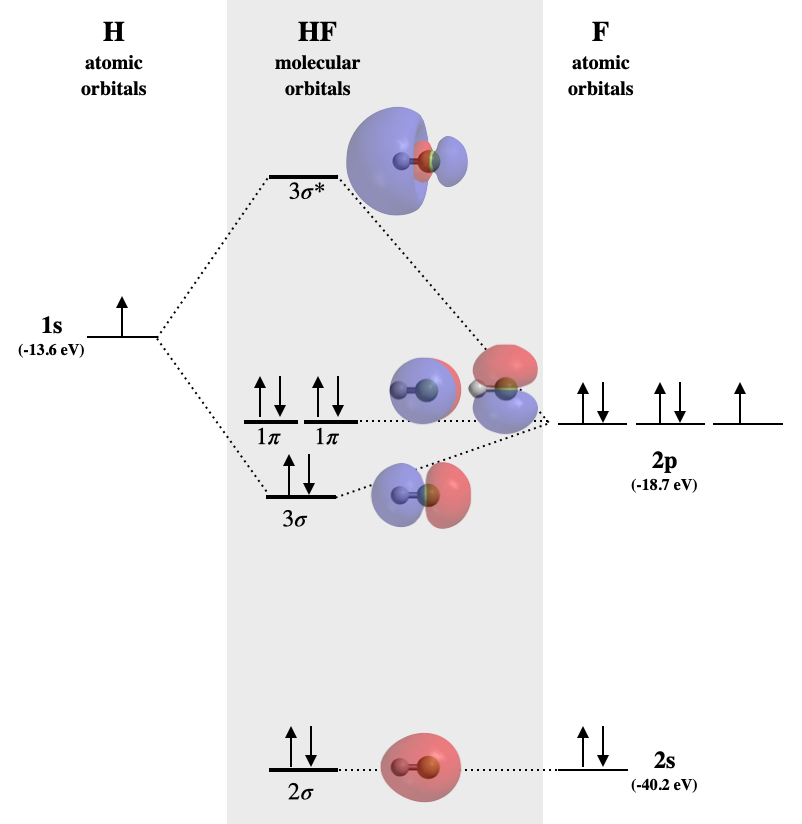
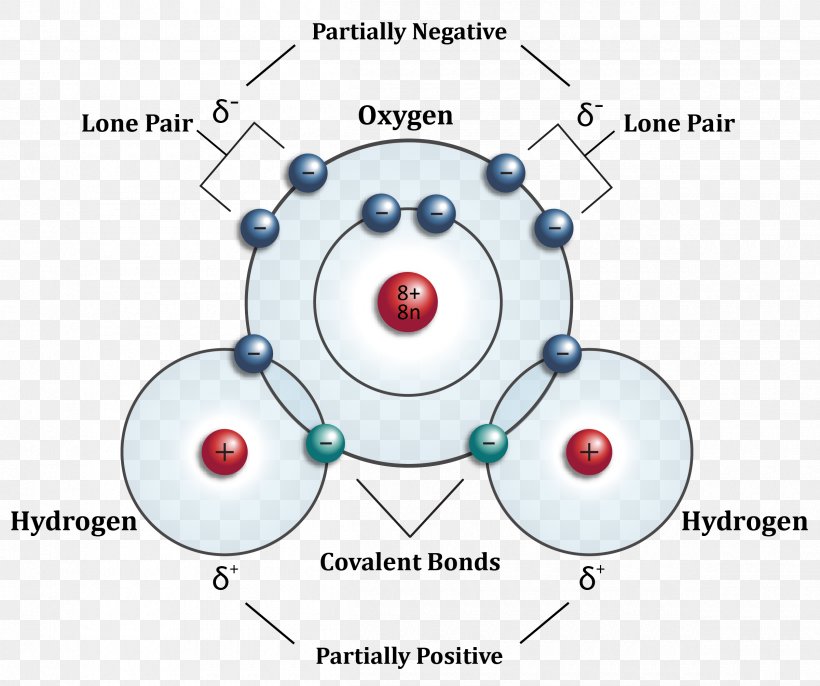
4.3.1 Polar bonds Chemistry LibreTexts
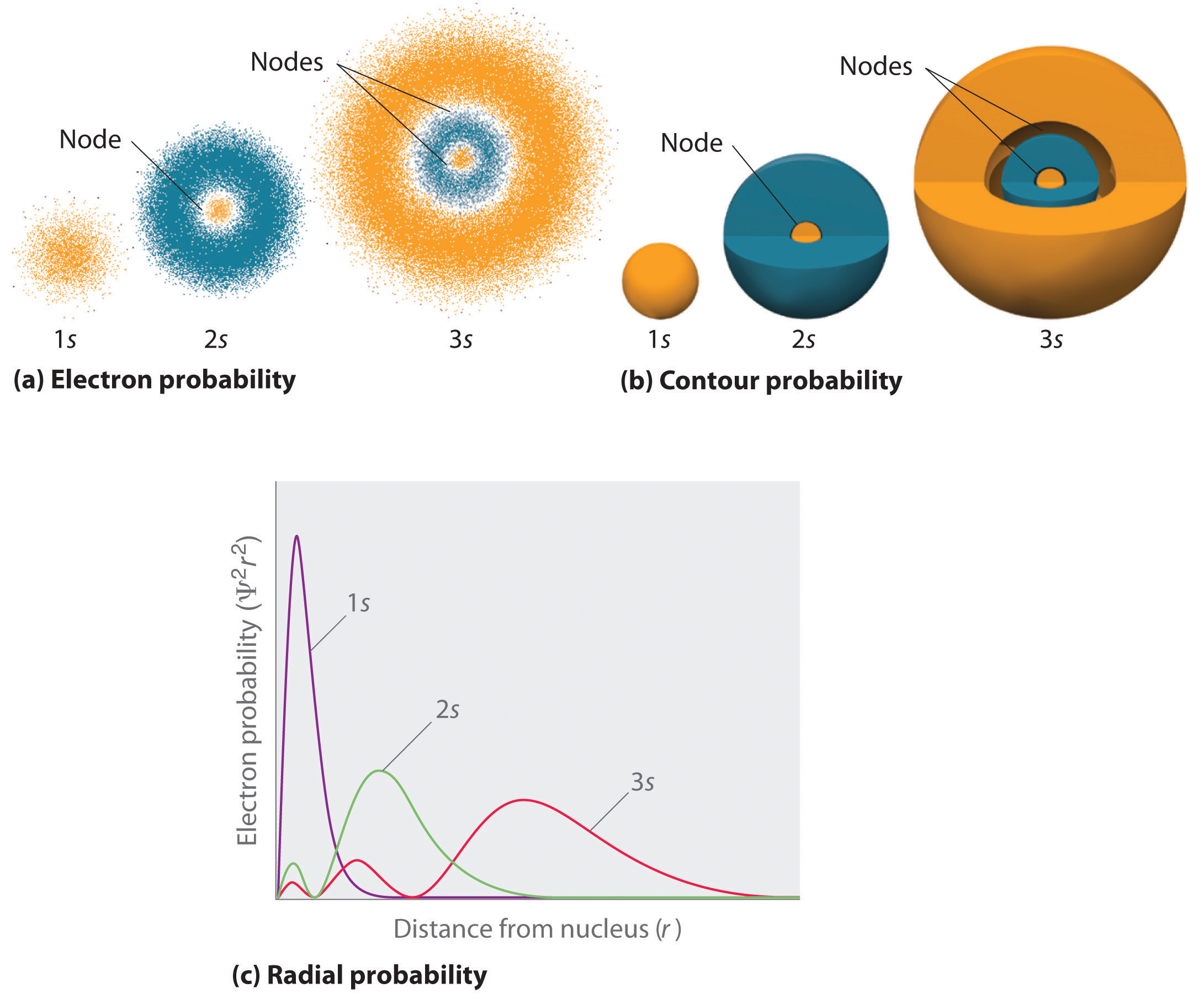
Atomic orbitals of the electron in a hydrogen atom at different energy levels. The probability of finding the electron is given by the color, as shown in the key at upper right. Electron properties
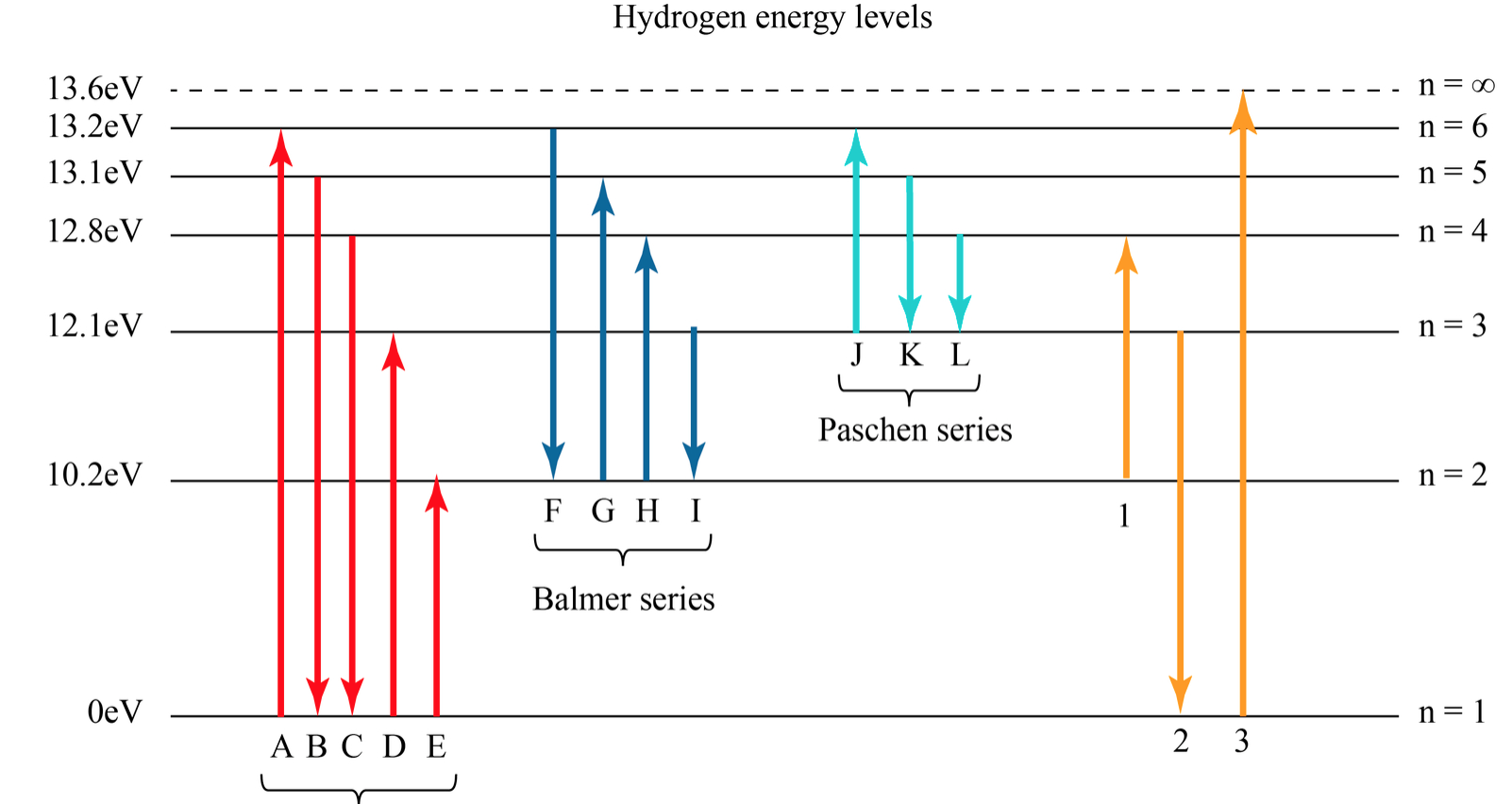
Solved Hydrogen Energy Level DiagramThe orbitals of
Looking at hydrogen, for example, its symbol, H, and name appear, as well as its atomic number of one—in the upper left-hand corner—and its relative atomic mass of 1.01. Image credit: modified from OpenStax Biology. An accessible version of the periodic table is available here.
Molecular Orbital Diagram Of Hydrogen Wiring Service
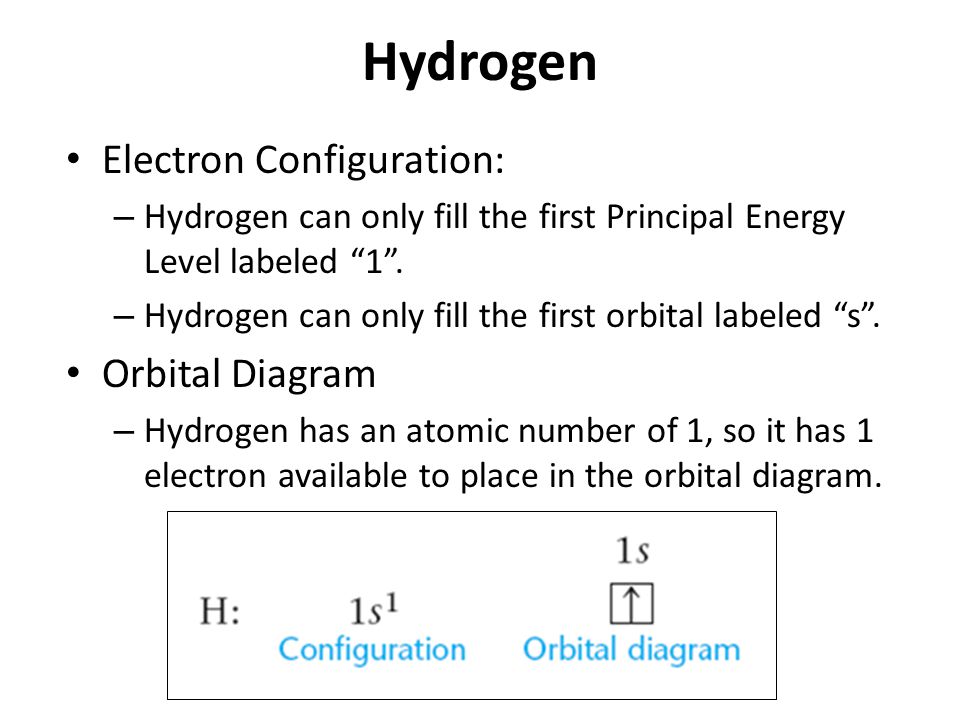
Here is a schematic orbital diagram for a hydrogen atom in its ground state: Figure \(\PageIndex{1}\): One electron in. From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number n and their value of l ( s , p , d , or f ), with the.

iodine orbital diagram BorisLayson
The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit (shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit]

Hydrogen Bonding Diagram
The hydrogen orbital diagram is a graphical representation of the electron configuration of the hydrogen atom. This diagram shows how the electrons in the hydrogen atom are arranged in different orbitals. Orbital is the region of space around the nucleus of an atom where electrons are found.
Energy Level Hydrogen Atom Bohr Model Hydrogen Spectral Series, PNG
YouTube 0:00 / 1:37 To write the orbital diagram for the Hydrogen (H) first we need to write the electron configuration for just H. To do that we need to find the number of ele.

Hydrogen Electron Orbitals visualization Web Education
Key points Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, n : E ( n) = − 1 n 2 ⋅ 13.6 eV
How Can You Find The Hydrogen Electron Configuration (H)
Figure \(\PageIndex{7}\): Orbital Energy Level Diagram for the Hydrogen Atom. Each box corresponds to one orbital. Note that the difference in energy between orbitals decreases rapidly with increasing values of n. In general, both energy and radius decrease as the nuclear charge increases. Thus the most stable orbitals (those with the lowest.
Chapter 2.5 Atomic Orbitals and Their Energies Chemistry LibreTexts
Hydrogen orbital diagram In the hydrogen orbital diagram, the 1s subshell contains a single electron. To draw the hydrogen orbital diagram, start by determining the number of electrons from the periodic table.

quantum mechanics How does the electron jump across "gaps" in its
Note that the smallest value of energy is obtained for n = 1, n = 1, so the hydrogen atom cannot have energy smaller than that. This smallest value of the electron energy in the hydrogen atom is called the ground state energy of the hydrogen atom and its value is. E1 = −E0 = −13.6eV. E 1 = − E 0 = −13.6 eV. 6.46.

hydrogen orbital diagram Eco Press
The diagram (not to scale) summarises the energies of the various orbitals in the first and second levels. Notice that the 2s orbital has a slightly lower energy than the 2p orbitals. That means that the 2s orbital will fill with electrons before the 2p orbitals. All the 2p orbitals have exactly the same energy. The electronic structure of hydrogen

Molecular Orbital Diagrams 101 Diagrams
When Hydrogen forms a covalent bond with another Hydrogen atom, H 2 is formed. Below are the corresponding atomic and molecular orbital diagrams for this element. To learn more about how to construct these diagrams for other elements, follow along with the steps below. Steps for Constructing an Orbital Diagram Atomic Orbital Diagrams
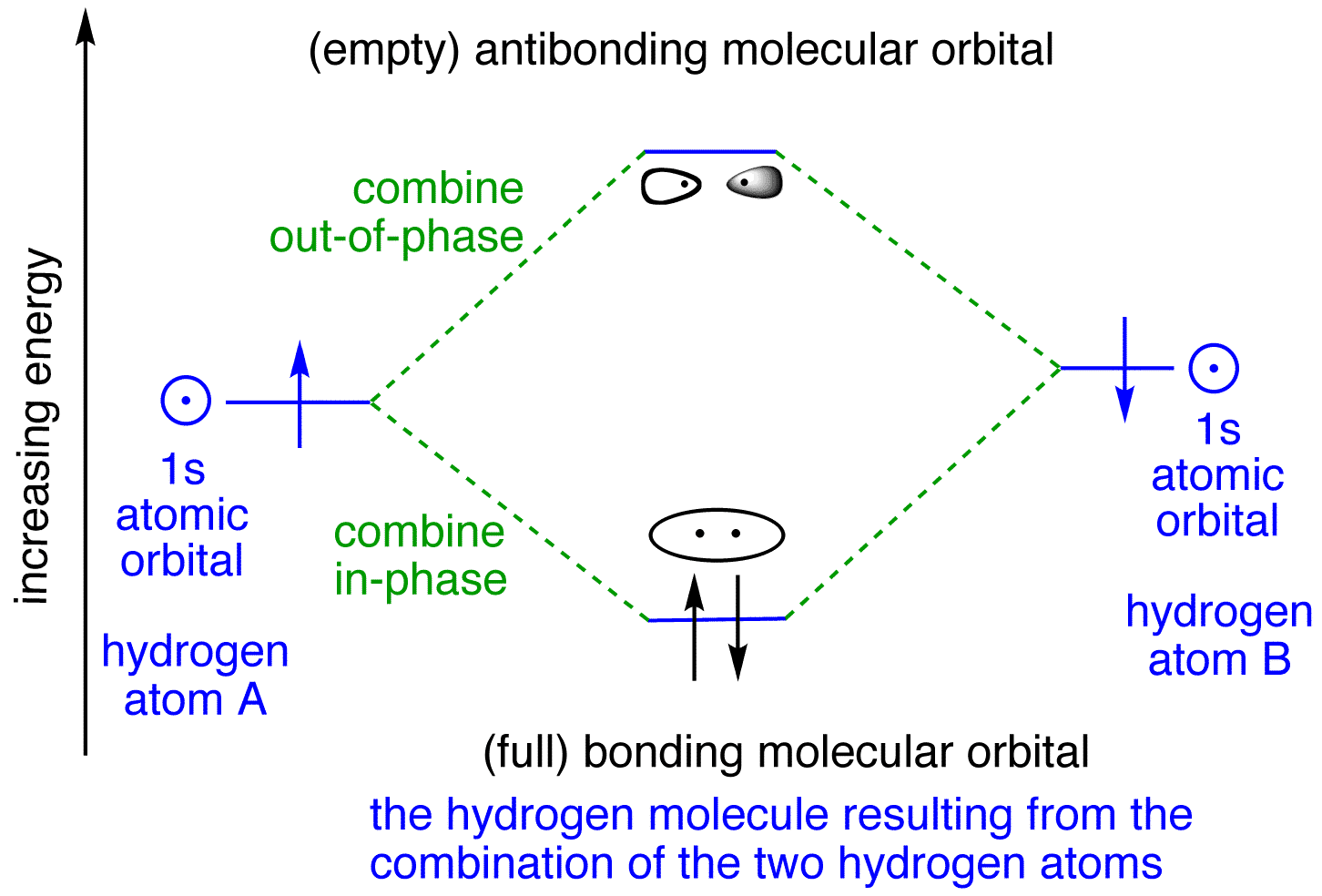
Molecular orbitals in Hydrogen
In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f. Figure 6.24 depicts how these two trends in increasing energy relate.
Hydrogen Atom Water Molecule Molecular Orbital Diagram, PNG
The diagram shows a cross-section through this spherical space. 95% of the time. Hydrogen only has one electron and that will go into the orbital with the lowest energy - the 1s orbital. Hydrogen has an electronic structure of 1s 1. We have already described this orbital earlier. Example 2: The electronic structure of carbon;