12 ka table YouTube

1 ka Table 1 ka Pahada math table 1 का पहाड़ा 1 का टेबल Todaysera
Table of Common Ka Values for Weak Acids Thomas Barwick/Getty Images By Todd Helmenstine Updated on February 03, 2020 K a is the equilibrium constant for the dissociation reaction of a weak acid. A weak acid is one that only partially dissociates in water or an aqueous solution. The value of K a is used to calculate the pH of weak acids.
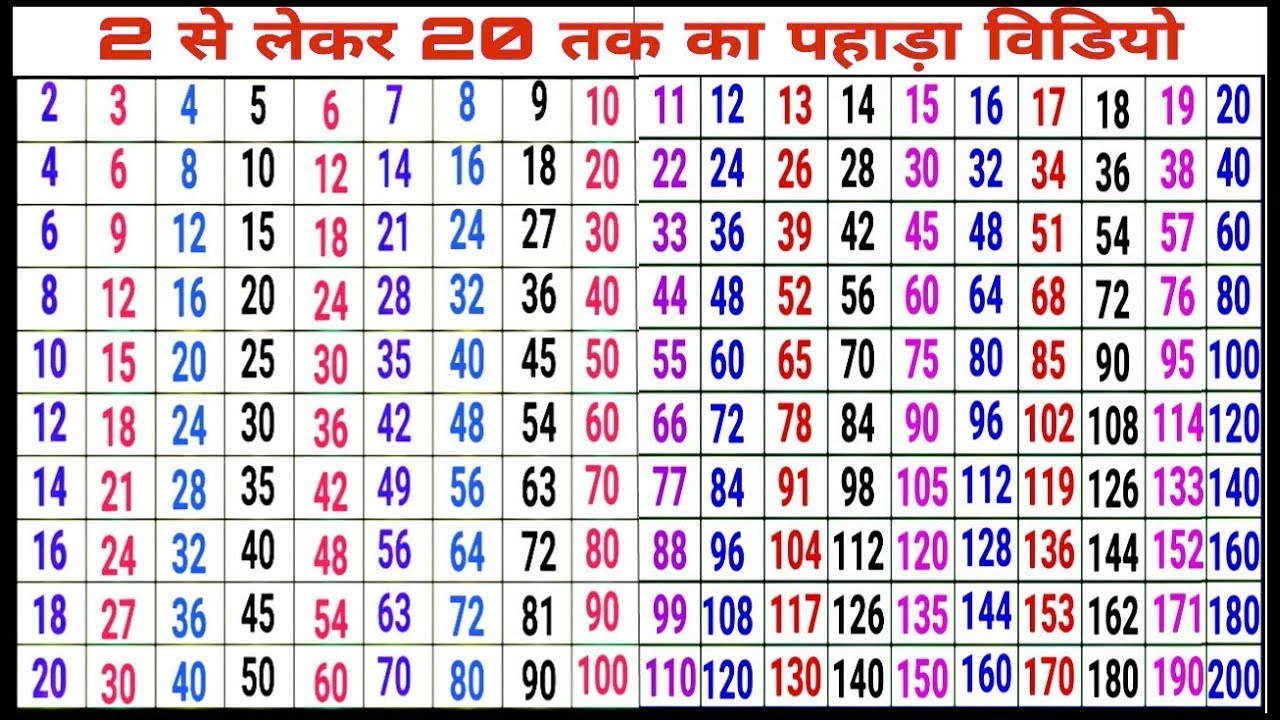
2 ka table. 2 Se 20 tak ka pahada video in english. YouTube
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.
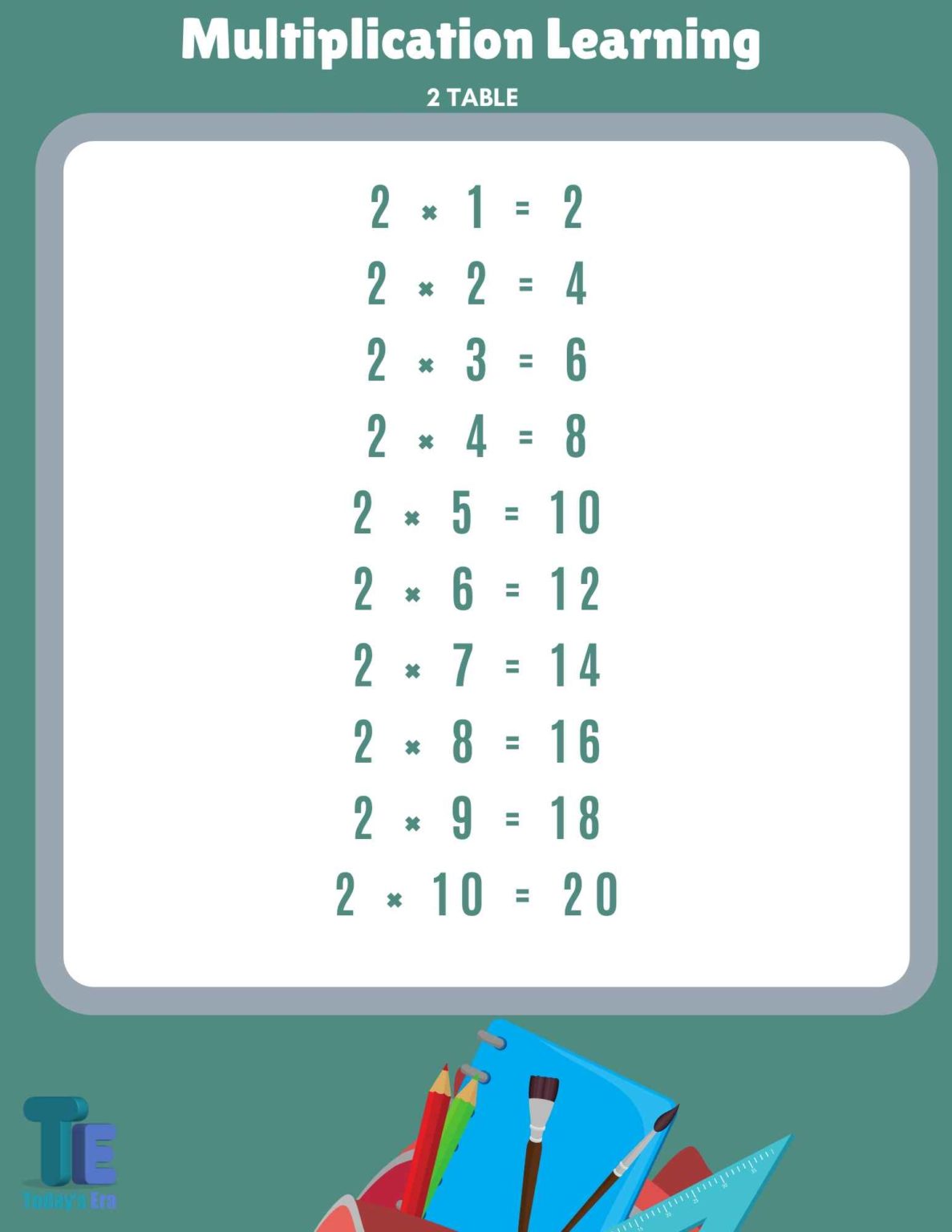
2 ka Table 2 ka Pahada Math Table 2 2 का पहाड़ा 2 का टेबल
Out of our three weak acids, hydrofluoric acid is the strongest, so it has the largest value for Ka, but notice it has the smallest value for the pKa. The lower the value for pKa, the more acidic your acid. 3.46 is lower than 4.74, and so hydrofluoric acid is more acidic than acetic acid. Up next: video. Learn for free about math, art, computer.

Ka Table Decoration Examples
Using an ICE table, we can find the pH of the solution when the sodium acetate is added: I: There is initially a 0.50M concentration of acetic acid.. The Ka value is found by looking at the equilibrium constant for the dissociation of the acid. The higher the Ka, the more the acid dissociates. Thus, strong acids must dissociate more in water.

2 se 100 tak ka table likh kar dikao Brainly.in
Table of 1 | table 1 2 | 1 ka table | Multiplication Table 1 #table | 1 time table | #multiplicationtable | 1x1 table | appendix table 1 | multiplication.

4 ka table YouTube
ka-table is "props - UI - action - reducer". (Thanks to Redux & Flux for the inspiration) ka-table UI is rendered according to props -> all changes are performed by dispatching an action -> previous props and action are passed to the kaReducer -> kaReducer generates new props ka-table easily integrates with Redux but also can be used without it
2 Ka Table Table Decorations
The values of \(pK_a\) and \(pK_b\) are given for several common acids and bases in Table 16.5.1 and Table 16.5.2, respectively, and a more extensive set of data is provided in Tables E1 and E2. Because of the use of negative logarithms, smaller values of \(pK_a\) correspond to larger acid ionization constants and hence stronger acids.

2 Ka Table Table Decorations
ka-table - React table component | ka-table IDEA props-UI-action-reducer ka-table UI is rendered according to props passed to the component. All changes are performed by dispatching an action, and kaReducer generates new props. This approach allows having full control over every change.
katable npm
Periodic Tables; TI-Programs; Chemistry Freeware; Acid Ka Values. Note: A K a of "very large" indicates a strong acid. Acid: Formula: Conjugate Base: K a: Perchloric : HClO 4 : ClO 4- Very large : Hydriodic. 1.3 x 10-10 : Hydrogen carbonate ion : HCO 3- CO 3 2- 5.6 x 10-11 : Hydrogen peroxide : H 2 O 2 : HO 2- 2.4 x 10-12 : Monohydrogen.
Fitfab 8 Ka Table
Ka Table. 1.5×10-10. Urea hydrogen ion NHCONH6.7×10-1. Zinc 2+ ion Zn2+2.5×10-10.

Information 46 ka table How to make 46 ka table ka table
Easy learning of Multiplication table of 1 | 1 table | one table | 1 x 1 = 1
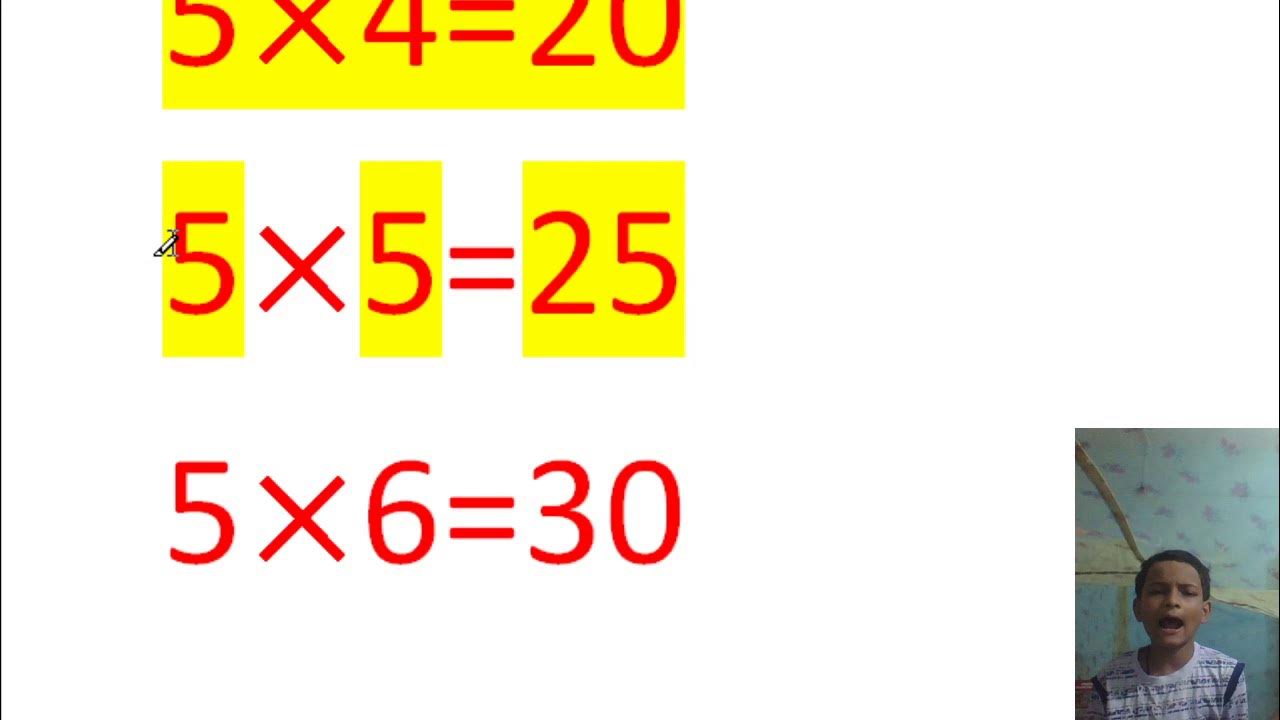
FIFTH KA TABLE YouTube
H 2 CO 3. 1) 4.3 × 10 -7. 2) 5.6 × 10 -11. citric acid. H 3 C 6 H 5 O 6. 1) 8.4 × 10 -4. 2) 1.8 × 10 -5. 3) 4.0 × 10 -6. oxalic acid.

2 Ka Table Table Decorations
Calculating a Ka Value from a Known pH Page ID The quantity pH, or "power of hydrogen," is a numerical representation of the acidity or basicity of a solution. It can be used to calculate the concentration of hydrogen ions [H +] or hydronium ions [H 3 O +] in an aqueous solution.

2 ka table 2 se 10 tak ka table pahdha video Hindi version multiplication tables table_ 2
Table of Acids with Ka and pKa Values* CLAS Acid. HA. A-Ka; pKa ; Acid Strength : Conjugate Base Strength . Hydroiodic; HI. I-Hydrobromic; HBr; Br-Perchloric; HClO; 4; ClO. 4-Hydrochloric. HCl. Cl-. (Kb > 1, pKb < 1). Conjugate acids (cations) of strong bases are ineffective bases. * Compiled from Appendix 5 Chem 1A, B, C Lab Manual and.

2 ka table Table of 2 2 का पहाड़ा tableof12 multiplicationtable पहाड़े kids_art YouTube
Bordwell pKa Table General Info pKa is an acid dissociation constant used to describe the acidity of a particular molecule. Its value is directly related to the structure of the given compound.

12 table up to 20. 12 ka table 20 Tak. 12 TABLE UP TO 20 STEPS. 12 KA PAHADA. 12 KA TABLE. YouTube
Table 1, 2 ka in English | 1 ka table, 2 ka table | Multiplication tables | Math#table_1_ka#2_ka_table #3_ka_table #math Subscribes: https://www.youtube.com.