Chemistry Balancing Equations Practice Worksheet Answer Key / Balancing

Identifying And Balancing Chemical Equations Worksheets Answ
2OH - → H 2 O + 1/2 O 2 + 2 e -. This is now balanced, but if you're one of those students that doesn't like fractions in chemical equations, or the question asks for whole number coefficients, just multiply by the denominator ( i.e. double everything): 4OH - → 2H 2 O + O 2 + 4e -.
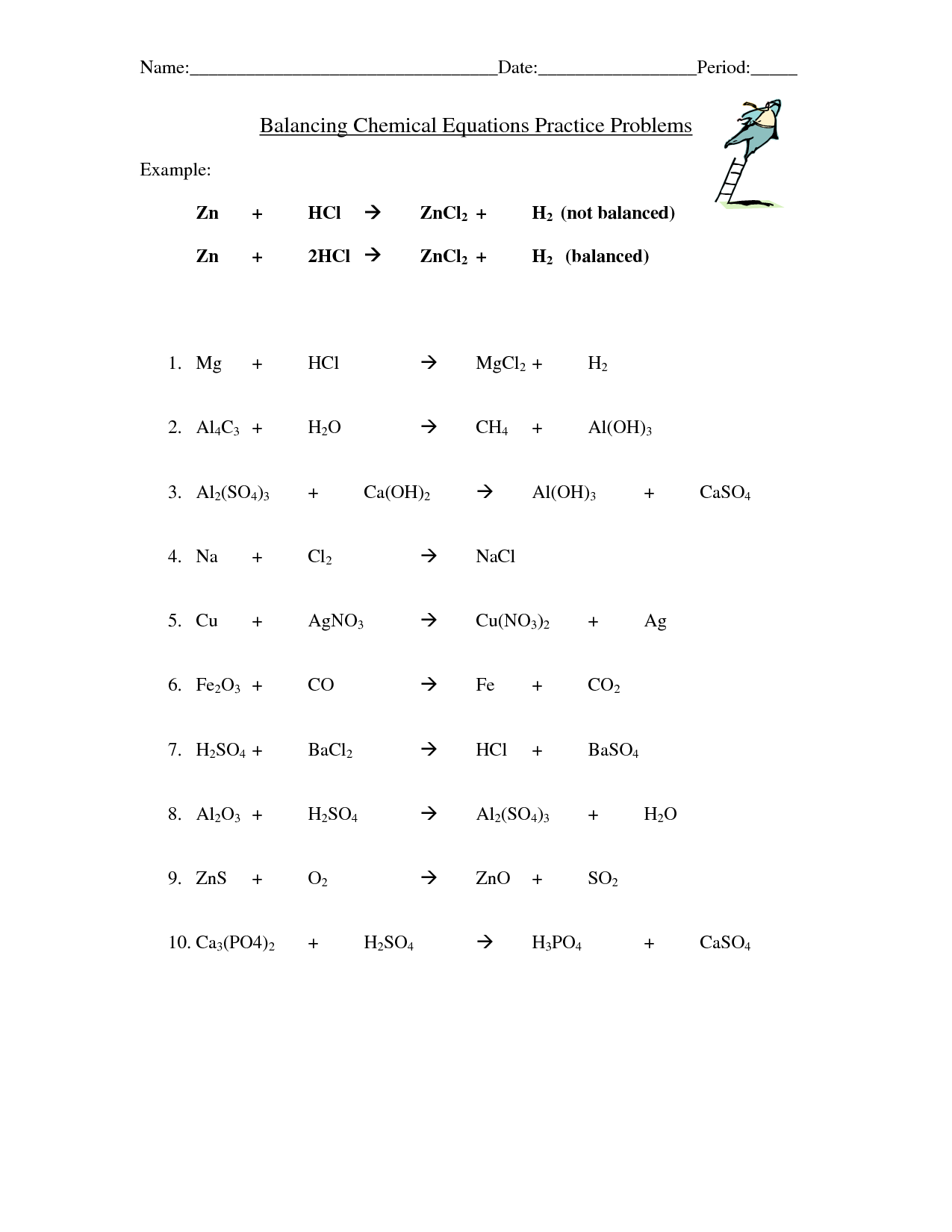
balancing chemical equation worksheet 1
This worksheet includes some rules and guidelines to help you balance chemical equations. Rules 1.) The formulas of the reactants and products cannot be changed, do not alter subscripts or charges. 2.) The only numbers that can be changed are the numbers indicating how many molecules or atoms, which are called coefficients. 3.)

Answer Key Balancing Chemical Equations Practice Worksheet With Answers
Balancing chemical equations requires practice. Once you've done it a few times, it becomes easier and easier. This balancing chemical equations practice sheet has ten more unbalanced chemical equations to solve. Download a PDF of this worksheet here. A PDF of the answer key is also available here.
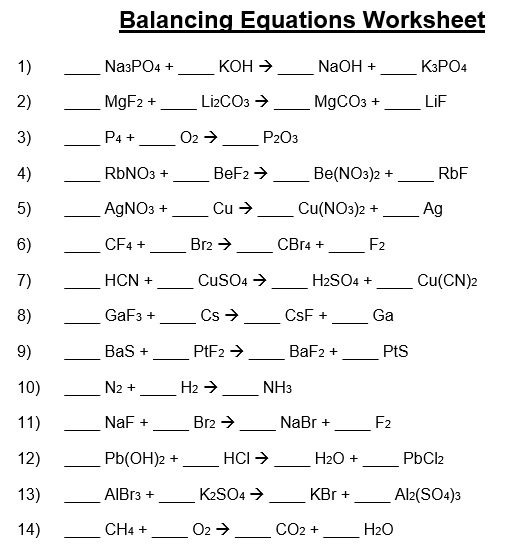
Free Balancing Chemical Equations Worksheets [With Answers] Best
Write the word equations below as chemical equations and balance: Zinc and lead (II) nitrate react to form zinc nitrate and lead. Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Sodium phosphate and calcium chloride react to form calcium phosphate and sodium chloride.

Balancing Chemical Equations Worksheet
Practice balancing chemical equations. % The current browser window is too small to render this simulation.. Exploration Series | Chemistry Simulations. Worksheet. Aa. Aa. Aa. Aa. Challenge Me. Feedback. FullScreen. Concepts. Tutorial. Assign to Class. Quick Assignments! You can directly assign a simulation to your classes and set a due date.

Chemistry Balancing Equations Practice Worksheet Answer Key / Balancing
Q1. A balanced chemical equation is in accordance with- Multiple proportion Reciprocal proportion Conservation of mass Definite proportions Correct Answer: (c) Law of Conservation of Mass Q2. The correct balanced equation for the reaction __C2H6O + __O2 → __CO2 + __H2O is- 2C 2 H 6 O + O 2 → CO 2 + H 2 O C 2 H 6 O + 3O 2 → 2CO 2 + 3H 2 O

49 Balancing Chemical Equations Worksheets [with Answers]
PROBLEM 5.1.1. 4. Write a balanced equation describing each of the following chemical reactions. Solid potassium chlorate, KClO 3, decomposes to form solid potassium chloride and diatomic oxygen gas. Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6.

12 Best Images of Balancing Chemical Equations Worksheet PDF
1 What is a Chemical Equation? 2 Balancing Chemical Equations Worksheets 3 Why is it Important to Balance the Chemical Equations? 4 Balancing Equations Worksheets with Answers 5 What are Different Types of Chemical Equations? 6 Balancing Equations Practice Worksheet 7 How to Balance a Chemical Equation?

Chemistry Balancing Chemical Equations Practice Worksheet Answer Key
Balancing chemical equations 1 Google Classroom Balance the following chemical equation: Mg (OH) 2 + HCl → MgCl 2 + H 2 O Note: All reactants and products require a coefficient of at least one. Stuck? Review related articles/videos or use a hint. Report a problem Do 4 problems

Balancing Chemical Equations Worksheet Pdf —
Balancing chemical equations (KS3/GCSE) - Questions © www.chemistrytutor.me 2018 Page 1 of 3 1. _O 2 + _NH 3-> _HNO 3 + _H 2 O 2. _O 2-> _O 3 3. _H
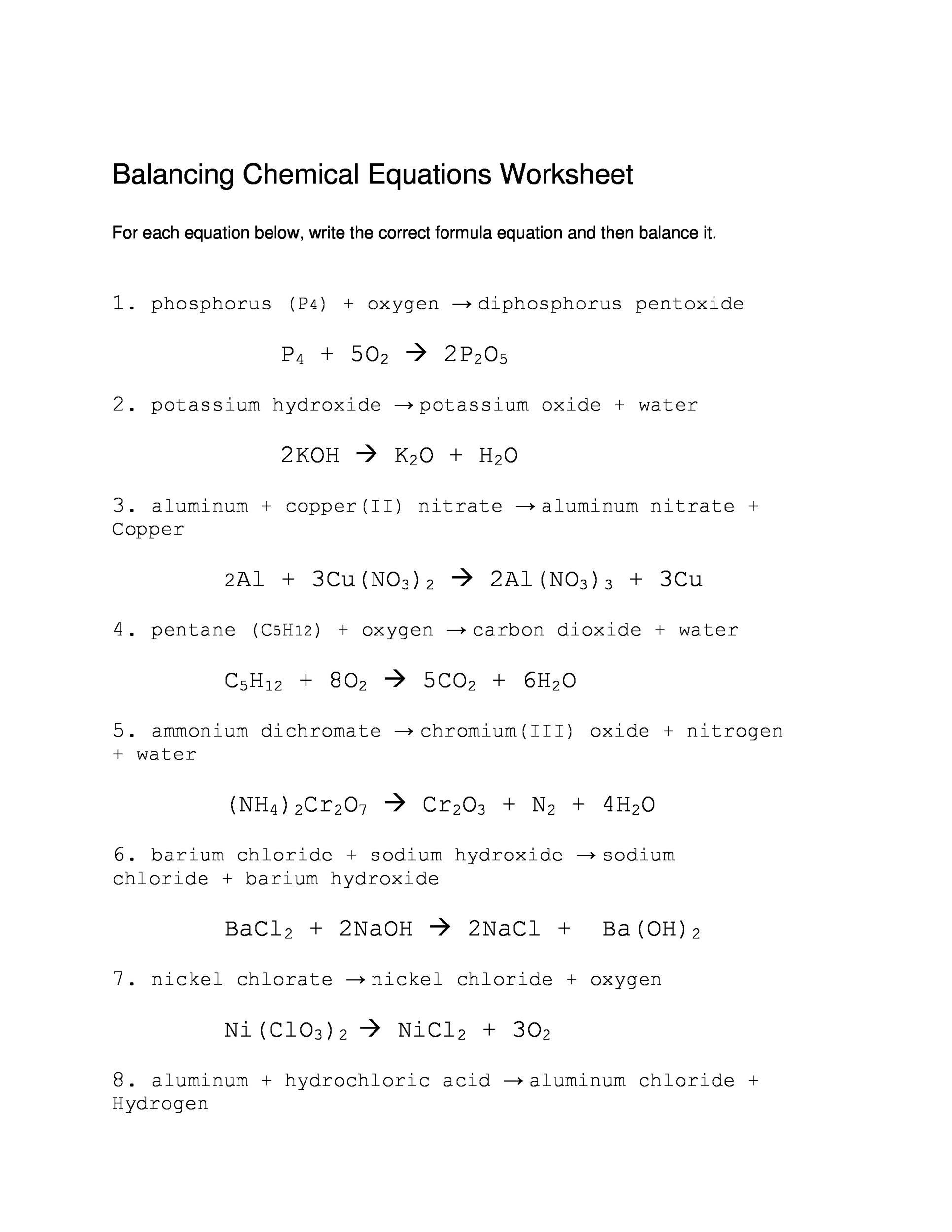
49 Balancing Chemical Equations Worksheets [with Answers]
This worksheet serves as a dynamic tool to reinforce the principles of conservation of mass and the fundamental laws that govern chemical transformations. Engage in an immersive learning experience as you work through real-world scenarios and discover the art of balancing chemical equations. The accompanying answer key ensures a self-paced.
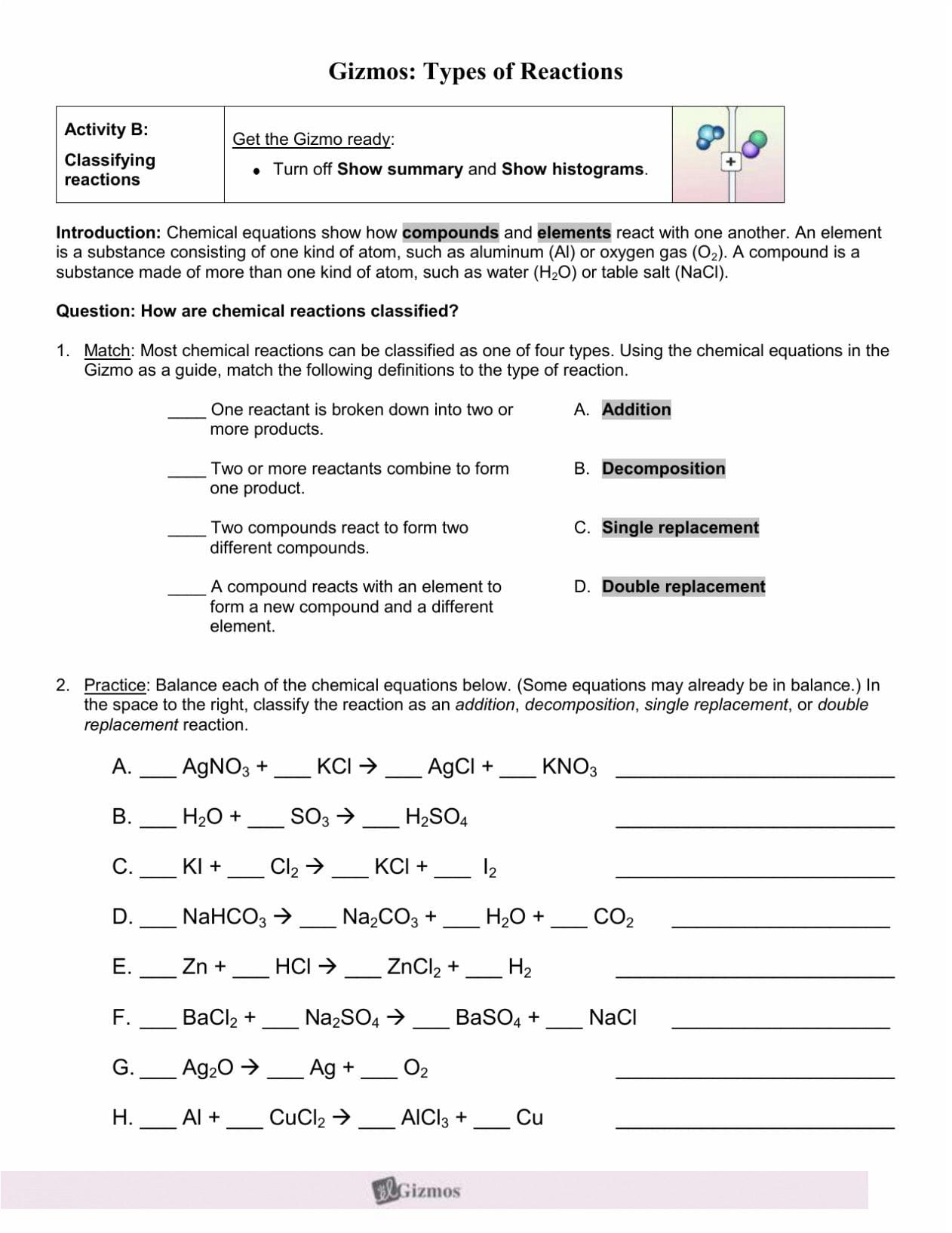
Balancing Chemical Equations Practice Worksheet —
in CuSO4 has an oxidation number of +6 So between 3CuS to 3 CuSO4 there will be a total increase of 3 x +8 = +24. This means N must decrease by -24 In HNO3 the oxidation number Of N is +5 and in NO it is +2. The change is -3 Therefore there must be 8 N's to decrease by -24 (8 x-3). So we must have 8HNO3 and 8NO.

49 Balancing Chemical Equations Worksheets [with Answers]
Balancing Chemical Equations - Worksheet #1 Balancing Chemical Equations - Answers #1 Balancing Chemical Equations - Worksheet #2 Balancing Chemical Equations - Answers #2 Balancing Chemical Equations - Worksheet #3 Balancing Chemical Equations - Answers #3 Balancing Equations - Worksheet #4 Balancing Equations - Answer Key #4

49 Balancing Chemical Equations Worksheets [with Answers]
Directions: First, balance each of the chemical equations below. Then, classify each reaction as synthesis, decomposition, single-replacement, or double-replacement. To earn full credit, write the words out when classifying. Balance the equation. ____ Sb + ____ Cl2 ____SbCl3 ____ Mg + ____O2 ____MgO

Chemistry Balancing Chemical Equations Worksheet 3 Answer Key
This balancing chemical equations worksheet has ten unbalanced equations to practice your skills. Either right-click and save the image or else download the PDF of the worksheet here. The worksheet prints on a standard sheet of printer paper. Balancing Chemical Equations Worksheet - Answer Key
49 Balancing Chemical Equations Worksheets [with Answers]
The ultimate goal for balancing chemical equations is to make both sides of the reaction, the reactants and the products, equal in the number of atoms per element. This stems from the universal law of the conservation of mass, which states that matter can neither be created nor destroyed.